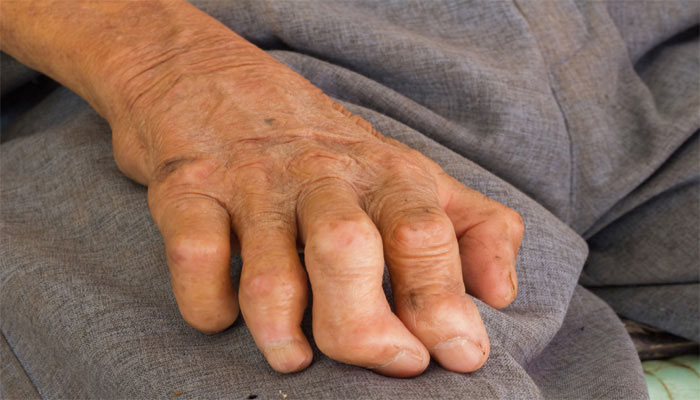
Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae and principally acquired during childhood or young adulthood. The skin, mucous membrane of the upper respiratory tract, and peripheral nerves are the major sites of involvement in all forms of leprosy. The clinical manifestations, natural history, and prognosis of leprosy are related to the host response, and the various types of leprosy (tuberculoid, lepromatous, etc.) represent the spectra of the host’s immunologic response (cell-mediated immunity).
Causes of Leprosy
The clinical spectrum of leprosy depends exclusively on variable limitations in the host’s capability to develop effective cell-mediated immunity (CMI) to M. leprae. The organism is capable of invading and multiplying in peripheral nerves and infecting and surviving in endothelial and phagocytic cells in many organs. Subclinical infection with leprosy is common among residents in endemic areas. Presumably the subclinical infection is handled readily by the host’s CMI response, Clinical expression of leprosy is the development of a granuloma; and the patient may develop a “reactional state,” which may occur in some form in >50% of certain groups of patients. The granulomatous spectrum of leprosy consists of
- a highresistance tuberculoid response (TT)
- a lowor absent-resistance lepromatous pole (LL)
- a dimorphic or borderline region (BB) and two intermediary regions:
- borderline lepromatous (BL)
- borderline tuberculoid (BT)
In order of decreasing resistance, the spectrum is TT, BT, BB, BL, LL.
Immunologic Responses Immune responses to M. leprae can produce several types of reactions associated with a sudden change in the clinical status.
Lepra Type 1 Reactions (Downgrading and Reversal Reactions) Individuals with BT and BL develop inflammation within existing skin lesions. Downgrading reactions occur before therapy; reversal reactions occur in response to therapy. Type 1 reactions can be associated with low-grade fever, new multiple small “satellite” maculopapular skin lesions, and/or neuritis.
Lepra Type 2 Reactions (Erythema Nodosum Leprosum, ENL) Seen in half of LL patients, usually occurring after initiation of antilepromatous therapy, generally within the first 2 years of treatment. Massive inflammation with erythema nodosum-like lesions.
Lucio’s Reaction Individuals with diffuse LL develop shallow, large polygonal sloughing ulcerations on the legs. The reaction appears to be either a variant of ENL or secondary to arteriolar occlusion. The ulcers heal poorly, recur frequently, and may occur in a generalized distribution. Generalized Lucio’s reaction is frequently complicated by secondary bacterial infection and sepsis.
Symptoms of Leprosy
- one or more hypopigmented (lighter than your normal skin color) skin lesions that have decreased sensation to touch, heat, or pain
- numbness or absent sensation in the hands and arms, or feet and legs
- skin lesions that do not heal after several weeks to months
Diagnosis
Made if one or more of the cardinal findings are detected: skin lesions characteristic of leprosy with diminished or loss of sensation, enlarged peripheral nerves, finding of M. leprae in skin or, less commonly, other sites.
Treatment
General principles of management include: eradicate infection with antilepromatous therapy, prevent and treat reactions, reduce the risk of nerve damage, educate patient to deal with neuropathy and anesthesia, treat complications of nerve damage, rehabilitate patient into society. Management involves a broad multidisciplinary approach including orthopedic surgery, ophthalmology, and physical therapy.
Antilepromatous Therapy:
Multidrug Regimens (Adult Doses)
Paucibacillary Disease (TT and BT)
Monthly, supervised Rifampin, 600 mg
Daily, unsupervised Dapsone, 100 mg
Duration 6 months; all treatments then stop
Follow-up after stopping treatment Minimum of 2 years with clinical exams at least every 12 months
Multibacillary Disease (LL, BL, and BB)
Monthly, supervised medication Rifampin, 600 mg Clofazimine, 300 mg
Daily, unsupervised medication Dapsone, 100 mg Clofazimine, 50 mg
Duration Minimum of 2 years, but whenever possible until slitskin smears are negative
Follow-up after stopping treatment Minimum of 5 years with clinical and bacteriologic examinations at least every 12 months
Therapy of Reactions
Lepra Type 1 Reactions Prednisone, 40 to 60 mg/d; the dosage is gradually reduced over a 2- to 3-month period. Indications for prednisone: neuritis, lesions that threaten to ulcerate, lesions appearing at cosmetically important sites (face).
Lepra Type 2 Reactions (ENL) Prednisone, 40 to 60 mg/d, tapered fairly rapidly; Thalidomide for recurrent ENL, 100 to 300 mg/d.
Lucio’s Reaction Neither prednisone nor thalidomide is very effective, Prednisone, 40 to 60 mg/d, tapered fairly rapidly.
Systemic Antimicrobial Agents Secondary infection of ulcerations should be identified and treated with appropriate antibiotics to prevent deeper infections such as osteomyelitis.
Orthopedic Care Splints should be supplied to prevent contractures of denervated regions. Careful attention to foot care to prevent neuropathic ulceration.
References